Tutorials: Electronics fundamentals (ELEFU)
Power and Energy
last updated: 2021-12-20
Quick links
Songs of this chapter:
The black eyed peas > The E.N.D. > Boom Boom Pow (full version).
The black eyed peas > The E.N.D. > Rock That Body.
On the album "The E.N.D." (Energy Never Dies) from the "The black eyed peas" we get interesting lyrics. In the full version of the song "Boom Boom Pow":
The Energy Never Dies
Energy cannot be destroyed
Or created
It always is
And it always will be
Such lyrics make the heart of a physics teacher pounding ;).
In another song ("Rock That Body") on the same album however we get:
"Electric shock, energy like a billion watts"
Don't remember this!!!! because the unit of the energy is surly not Watt. In more than 50 % of all newspaper articles units of energy and power are not correct. Hopefully we can get it right in this chapter :).
Songs of this chapter:
John Lennon > Power to the people
As seen in the chapter about Ohm's law we can calculate the a constant electrical power in a DC circuit with the following formula:
By substituting Ohm's law we get two derived formulas:
Here a wheel with all the possible conversions of the formulas:
As the power drawn from a circuit mostly changes over time we must difference between instantaneous power and average power. For the instantaneous power we use:
Power is the rate, per unit time, of doing work or transferring heat.
The symbol for the power is P. The SI unit is the J/s (joule per second) or Watt.
This gives us another formula for constant power:
Mostly power is not constant. We calculate the average power with:
For exact instantaneous power we use the limit from the time interval approaching zero:
Peak power is the maximum value the instantaneous power of a machine can have. If we name the power of a wind power plant or a photovoltaic power plant we speak of the peak power of the machine. Sometimes the peak is added to the unit (e.g. 4KWp).
Quantities of power
| wrist watch | 0.02 W |
| human (average power) | |
| personal computer | 500 W |
| average power of a cyclist during TDF | 250 W |
| peak power of a cyclist during TDF | 600 W |
| average power of a horse | 500 W |
| unit horse power (lift 75 kg 1 m in 1 s) | 735.5 W |
| power of the sun on a shiny day in Europe | 1000 W |
| LAM solar car Râ-le-Sol (1992) | 12 kW |
| Nissan Leaf 2020 (EV) | 110 kW (147 hp) |
| electric locomotive | 3 MW |
| biggest wind power plant 2020 | 12 MW |
| all Luxembourgian wind power plants 2020 | 153 MW |
| all Luxembourgian pv plants 2020 | 187 MW |
| one machine in Vianden (SEO) | 100/200 MW |
| Luxembourgian peak power demand 2019 (CREOS) | 0.829 GW |
| pumped storage hydro power plant Vianden (SEO) | 1.3 GW |
| nuclear power plant Cattenom: 4x1.3GW | 5.2 GW |
Just do it Power 1
- Measure the voltage and the current needed by an Arduino Uno, an MH-ET LIVE D1 mini ESP32 and a Raspberry Pi 3 B+. Calculate the power needed.
With climate change it is important to reduce our energy consumption. The electrical efficiency of all our electronic devices must be increased!
The efficiency of a device is defined as useful power output divided by the total electrical power input.
The symbol for the efficiency is the small Greek letter η. Efficiency is a dimensionless number and is often expressed in percent.
Just do it Power 2
- We will re-look at a voltage divider with the following data:
U = 5 V, R1 = 1.8 kΩ, R2 = 3.3 kΩ.
Calculate the efficiency for the following loads:100 Ω, 220 Ω, 560 Ω, 1 kΩ, 1.8 kΩ, 3.3 kΩ, 6.8 kΩ, 15 kΩand27 kΩ. Use Libreoffice Calc or Excel to calculate the efficiency. Document the table. - Draw a graph containing
Pout = f(RL)andη = f(RL). - What are your conclusions regarding the output power and the efficiency?
- Search for an electronic component that reduces 5 V to 3.3 V on your ESP32 board. Note its denomination and search for the corresponding data-sheet.
- A similar integrated circuit, often used on other boards is the
MCP1825. Look at the data-sheet and read the chapter about power calculations (5.2). Calculate the efficiency for the power dissipation example (5.3.1).
Just do it Power 3
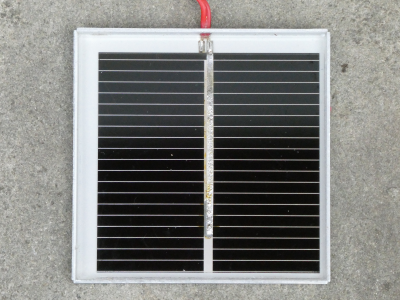
- Solar cells are with wind power plants the two renewable power generators that could save the planet. Solar cells are also interesting for energy harvesting for low power IoT-devices.
A single illuminated cell has a voltage of about 0.5 V. The voltage can be increased by connecting more cells in series. The output current depends on the surface area of the cell and the amount of light. Measure the dimensions of your cell and calculate the area in m². - Calculate the output power of your cell under a shiny sun (1000 W/m²) when you suppose an efficiency of 15 %.
- The cell gives her maximum of power only to a specific load. This operating point is called Maximum Power Point MPP and power inverters try always to adapt the load in such a way to reach this MPP. Measure the voltage and current of your cell for the following loads:
0 Ω, 3.3 Ω, 6.8 Ω, 10 Ω, 12 Ω, 15 Ω, 18 Ω, 22 Ω, 27 Ω, 33 Ω,47 Ωand1 kΩ.
Tip: Place your cell close to an LED lamp and fix it with tape during the measurement.
Use Libreoffice Calc or Excel to calculate the power. Document the table. - Draw your circuit (including current and voltage arrows!). Research the symbol for a solar cell (german: Photozelle).
- Draw a diagram with two curves and two y-axes:
U = f(I)andP = f(I). Which load gives us the MPP? - Why you can't reach exactly 0 V when short circuiting your solar cell?
Songs of this chapter:
The Bangles > Different Light > Manic Monday.
No pain, no gain :).
A force does work if acting on a body, there is a displacement of the point of application in the direction of the force. Work transfers energy from one place to another, or one form to another.
For mechanical work:
If a constant force F acts on an object while the object is displaced a distance s, (force and displacement are parallel to each other) the work done on the object is the product of F and s.
If the force and the displacement are in the same direction, the work is positive. If the force and the displacement are in opposite directions (e.g. braking a car) the work is negative.
The unit Joule is equivalent to the newton-meter and the watt-second.
The symbol for work is W. The SI unit of work is the joule (J) (work expended by a force of one newton through a displacement of one meter) or watt-second (W⋅s).
The newton-meter (N⋅m) is sometimes used for work, but this can be confused with the same unit newton-meter, which is the measurement unit of torque.To avoid confusion joule should be used. Non-SI units of work include the kilowatt-hour and the horsepower-hour.
And as already seen we can calculate the work by multiplying power and time:
If work is done, energy is transferred to an object. Energy and work have the same units!
We can't live without energy, without food, without sun! Because energy in Europe is cheap and obtainable almost immediately and everywhere we often forget it's importance.
Energy is the ability to do work.
Energy is work that is stored.
If we lift a book from the floor (initial state, potential energy E1) to a table, the finite state is the potential energy on the table E2. Positive Work was done to the system by lifting the book. The system was energized (energy input). If we push the book from the table the system itself performs work (negative sign, energy output).
The symbol for energy is E. The SI unit is the joule (J) or watt-second (W⋅s)
For technical use it is important to be able to change the form of energy.
Different forms of energy:
We distinguish different forms of energy:
Mechanical energy: Kinetic energy of moving a mass, the potential energy stored by an object's position in a force field (gravitational, electric or magnetic) and the the elastic energy stored by stretching solid objects.
Chemical energy: Breaking or making of chemical bonds involves energy, which may be either absorbed or evolved from a chemical system. Examples: Burning food or coal, energy stored in batteries.
Radiant energy: Is the energy of electromagnetic and gravitational radiation. Carried by light (sun) but also in electromagnetic radiation with other frequencies. Examples: Radiometry, solar energy, heating (microwave), lighting, telecommunications.
Nuclear energy: It binds nucleons to form the atomic nucleus (and nuclear reactions).
Two special forms are the thermal energy (heat) and the electrical energy:
Thermal energy: The microscopic motion of particles gives heat. At 0 Kelvin = -273,15 °C we have no more motion and the thermal energy is zero. Heat is a form of disordered equivalent of mechanical energy, and has a special role to play because each time we convert one energy form to another a part of the converted energy will be heat. Mostly heat at a low temperature level, so it can not be used in our system. We think of heat as a loss in energy (not true because of our law of conservation of energy). We can convert most energy forms completely to heat, but reversely it is not true.
Electrical energy: Electrical energy is the energy caused by electrical charge. In it's static (potential) form we have this energy in a charged capacitor. Mostly it will be kinetic energy in the motion of charge. Charge in motion will always generate magnetic energy, so it is better to talk about electromagnetic energy. Energy has a special role to play because it's a very precious form of energy. It's transport can be done with little losses, it is relatively easy to store and it can be easily converted in another type of energy (heat, light, motion, etc.).
Quantities of energy
| 1 eV (electron-volt) | 1.6·10-19 J | 4.44·10-23 Wh |
| working person (8 h) | 3 MJ | 0.833 kWh |
| burning 1 kg of brown coal | 8.5 MJ | 2.4 kWh |
| burning 1 kg of dry wood | 14.7 MJ | 4.1 kWh |
| 1 kg TNT | 15.2 MJ | 4.2 kWh |
| 1 kg chocolate spread | 22.3 MJ | 6.2 kWh |
| 1 kg 1 kg diesel fuel (1.2 l) | 42.65 MJ | 11.8 kWh |
| 1 kg hydrogen (11.1 m³) | 120 MJ | 33.3 kWh |
| annual production PV Luxembourg 2020 | 576 TJ | 160 GWh |
| annual production wind Luxembourg 2019 | 1.26 PJ | 351 GWh |
| 1 kg uraniumdioxid (3.2%) | 2.46 PJ | 683 GWh |
| annual consumption electricity Luxembourg 2020 | 23,6 PJ | 6543 GWh |
| annual consumption Luxembourg 2018 | 186.6 PJ | 51.8 TWh |
| annual consumption world 2019 | 622.8 EJ | 173 PWh |
| annual solar energy on earth | 5.6·1024 J | 1555 EWh |
| daily radiation sun | 3.3·1031 J | 9.17·1024 kWh |
| annual radiation sun | 1.2·1034 J | 3.3·1027 kWh |
| total energy sun | 1045 J | 2.77·1038 kWh |
Just do it Energy 1
- Calculate the area needed to produce the whole energy of one year (annual consumption world) with solar panels. With an efficiency of 20 % we get EPV = 200 kWh/a·m² (a in the formula states for annual (per year)). Express the number in km² and in percent of the planets area.
- If you need 3000 kcal (1 kcal = 4187 J) per day of energy, you could supply this whole energy with chocolate spread. How many kg you have to eat per day?
Law of conservation of energy (wiki)
In a closed system, the amount of energy is fixed. You can't create any more energy inside the system or destroy any of the energy that's already in there. But you can convert the energy you have from one form to another (and sometimes back again).
It's important to be able to convert energy from one form to another. The law of conservation of energy says, that no energy is lost, which contradicts on first sight our everyday experience. If we use 1 kWh of electrical energy to lighten our house, we will get only about 0.15 kWh of radiant energy. 0.85 kWh thermal energy are losses in our eyes. This is true in summer as we can't use the resulting heat. In winter we will heat our home, and the thermal energy is not lost, as it has not to be produced by our heating installation.
If we speak of energy consumption, there is no consumption but a degradation of energy from a high order energy (e.g. electric energy) to a low order energy (heat at low temperature level).
Energy transformation is done by energy conversion machines. They convert one energy form to another. Often they need more than one step to reach the final form.
Just do it Energy 2
- Try to find direct converting machines for the following energy forms (fill the table and pay attention to the direction):
| ↗ | mechanical | electrical | chemical | thermal | |
| mechanical | |||||
| electrical | |||||
| chemical | battery | ||||
| thermal |
The efficiency (η) of energy conversion is the ratio between the useful output of an energy conversion machine and the input. It is the same as for the power because in a ratio the time can be eliminated.
Often the conversion from one form of energy to another will need more than one step. We get a chain of energy conversions.
The total efficiency can be calculated with:
Her are some examples of efficiencies of energy conversion machines. The values are approximately because the efficiency depends heavily on the operating point.
Examples of efficiencies of energy
| Conversion process | Energy efficiency |
|---|---|
| Electric heater | 100 % |
| Big electric generator | 95–99 % |
| Big electric motor | 90–99 % |
| Switched-mode power supply | 80-96 % |
| Lithium-ion battery | 80–90 % |
| Solar collector | 80 % |
| Little electric motor | 58 % |
| Fuel cell | 60 % |
| Electrolysis of water | 60 % |
| Gas turbine plus steam turbine (combined cycle) | 58 % |
| Gas turbine | 40 % |
| Diesel engine | 40 % |
| World Electricity generation (net) | 33 % |
| Gasoline engine | 30 % |
| Light-emitting diode (LED) | 26 % |
| Solar cell | 25 % |
| Muscle | 20 % |
| Fluorescent lamp | 14 % |
| Photosynthesis | 1-6 % |
| Incandescent light bulb | 1–2 % |
Just do it Energy 3
- Calculate the efficiency of a coal power plant if the efficiency of the firing chamber is 90 %, the efficiency of the turbine is 45 % and the efficiency of the generator is 98 %.
- The efficiency of cars with combustion energy is under 30 %.
As the efficiency of an electric car with batteries is about 90 %, we use 90 % of renewable energy if we power the car with solar energy.
Hydrogen has to be produced with electrolysis of water or with fuel cells. We can do this also from renewable energy (e.g. solar energy). Hydrogen cars can drive with fuel cells and electric motors or with a hydrogen combustion motor (no fuel cell). Calculate the overall efficiency for both solutions for a hydrogen car.
For IoT devices we often use chemical batteries to get energy.
First we must distinguish between primary batteries which are used once and then discarded and secondary batteries that can be discharged and recharged multiple times.
In primary batteries like alkaline batteries the electrode materials are irreversibly changed during discharge. They can be used only once.
In secondary batteries the original composition of the electrodes can be restored by reverse current. They are also named rechargeable battery or accumulator. Today we often use lithium-ion batteries because of their high specific energy (energy by unit mass).
The capacity of a battery is the amount of electric charge it can deliver at the rated voltage. It is normally expressed in ampere-hour (A·h). The capacity marked on a cell is usually measured during 20 hours at 20 °C on a new battery. The current delivered during this 20 hours must be such that the cell remains above a specified terminal voltage per cell.
The voltage of the battery declines steadily during use, so the total usable capacity depends on the cut-off voltage of device. Devices using per example alkaline cells (1.5 V) can stop functioning at 1.4 V. Other devices still work at 1 V. So the capacity of a battery depends on multiple factors, including battery chemistry, the load (current), the required terminal voltage, the storage period, the age of the battery, ambient temperature e.t.c..
If batteries are not used for a long time they discharge themselves and lose capacity due to irreversible side reactions (internal self-discharge). Also when batteries are recharged, the capacity is reduced a little bit. After enough recharges, in essence all capacity is lost and the battery stops producing power. The number of recharges before a battery is useless is named load cycle or charge cycle.
Discharging the battery at a low rate (current) results in a higher capacity than at a higher current.A battery rated at 3.4 A·h for a 20-hour discharge will not deliver a current of 1.7 A for two hours, but less.
Just do it Energy 4
- Our rover uses energy from AA alkaline batteries.
The capacity from such chemical batteries depends on the load. The effective capacity of an AA primary cell might be 3000 mAh at low drain (little current). At a high current of 1 A the capacity could be as little as 700 mAh.
For our calculations we suppose a capacity of 2000 mAh at 0,18 A (Wifi only) and 1400 mAh at 0.5 A (rover at full speed).
How long the rover could be switched on without moving and how long could he drive at full speed? - You have a fully charged Lithium battery with a marking of 3400 mAh. How long could we run the rover at full speed (0.5 A) if we get 80 % of the marked capacity at that current. To spare the battery we will discharge only down to 15% of the marked capacity!
Equivalent circuit diagram for batteries
We can think of a battery as an ideal voltage source in series with an internal resistance.
When we measure the voltage of a battery without load (RL = ∞) we get the open circuit voltage U0.
With load a current is flowing in the circuit, generating a loss of voltage on the inner resistance of the battery. There is a drop of the battery voltage proportional to the current.
For a short circuit (RL = 0) the battery voltage is zero (Ubatt = 0·I). The open circuit voltage equates the voltage on the internal resistance U0 = URi. We can calculate the internal resistance with:
or if we get two working points:
Just do it Energy 5
- We want to draw a diagram for our alkaline batteries U = f(I).
Don't use the lithium batteries!!
It will be a straight line because we assume the internal resistance is ohmic. To draw a straight line we need two points. The first point will be the open circuit voltage (I = 0). Take one of the AA alkaline cells and measure the open circuit voltage. For the second point we measure the short circuit with our Ammeter (U = 0). Do this only during a very short time (1 second) and use the 10 A range!! Draw the requested diagram. - Calculate the inner resistance.
- We want to confirm the linearity of our diagram, and want to measure one supplementary point at 0.5 A. Calculate the needed load (RL). Try to use an appropriate RL (use series and/or/parallel circuits if needed). Make sure your resistor is suitable for the heat output. Normal resistors can only handle 0.25 W! Measure the voltage and the current and add the point to your diagram.
Tip: Measure current and voltage separately and use the 10 A range on your ammeter. The inner resistance of the ammeter's the 400 mA range is too high! Also use short wires to reduce the resistance of the wires. - Calculate the efficiency of the battery with a load that draws 0.5 A. Where do the losses occur?
Just do it Energy 6
- Measure the open circuit voltage of a fully charged lithium ion cell (I = 0). Measure a second working point (voltage and current) with a load of 12 Ω/10 W. Calculate the internal resistance of the lithium ion battery and the theoretical short circuit current. Calculate the efficiency of this battery with a load of 12 Ω.
Just do it Energy 7
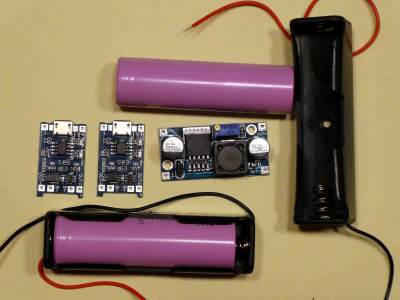
- We want to power the rover with rechargeable lithium ion batteries instead of the polluting primary cells. One lithium ion cell has a nominal voltage of 3.6 V. So we need two cells in series which give us 7.2 V. To get the needed voltage of 6 V we use an adjustable DC-DC step-down converter (board in the middle of the picture ). These little breakout boards (43x21x14mm³) use an LM2596 chip, convert 3-40 V to 1.5-35 V and can deliver up to 3 A. The input voltage must be 1.2 V higher than the output voltage! They have also over-temperature and short-circuit protection.
We want to measure the efficiency of the board at 0.18 A (only Wifi) and at 0.5 A (rover full speed). For this we will use resistors instead of the rover. Calculate the resistances and the power for the two resistors we need. - We will use a power supply instead of the lithium batteries to deliver 7.2 V to the DC-DC step-down converter. This reduces the risk of burning down the school. Measure the input and output voltages and currents for the two loads, and calculate the efficiencies.
